How Do You Know Charge of Transition Metals
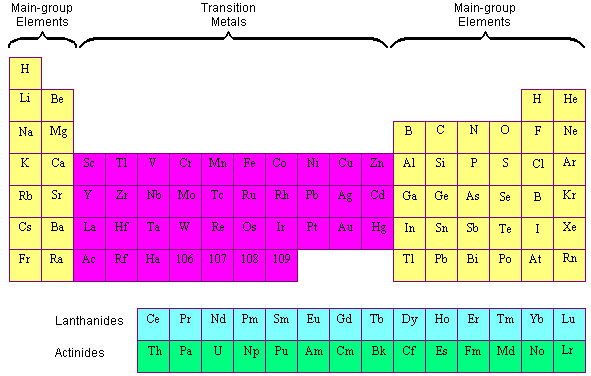
Transition Metals Position of Transition Metals in the Periodic Table The elements in the periodic table are often divided into four categories: (1) chief grouping elements, (two) transition metals, (3) lanthanides, and (iv) actinides. The main group elements include the active metals in the 2 columns on the extreme left of the periodic table and the metals, semimetals, and nonmetals in the six columns on the far right. The transition metals are the metallic elements that serve as a bridge, or transition, between the two sides of the table. The lanthanides and the actinides at the bottom of the table are sometimes known as the inner transition metals because they have diminutive numbers that fall betwixt the first and second elements in the last two rows of the transition metals. Transition Metals vs. Main-Group Elements At that place is some controversy almost the nomenclature of the elements on the boundary between the main group and transition-metal elements on the right side of the table. The elements in question are zinc (Zn), cadmium (Cd), and mercury (Hg). The disagreement about whether these elements should be classified as principal group elements or transition metals suggests that the differences between these categories are not clear. Transition metals are like main group metals in many ways: They wait like metals, they are malleable and ductile, they conduct heat and electricity, and they grade positive ions. The fact the ii best conductors of electricity are a transition metal (copper) and a principal group metal (aluminum) shows the extent to which the physical properties of main group metals and transition metals overlap. In that location are also differences betwixt these metals. The transition metals are more than electronegative than the main grouping metals, for example, and are therefore more likely to form covalent compounds. Some other difference betwixt the main group metals and transition metals can be seen in the formulas of the compounds they class. The main group metals tend to course salts (such as NaCl, Mg3N2, and CaS) in which there are merely enough negative ions to residue the charge on the positive ions. The transition metals form similar compounds [such as FeCl3, HgI2, or Cd(OH)two], but they are more likely than master grouping metals to form complexes, such equally the FeClfour -, HgIfour 2-, and Cd(OH)4 two- ions, that take an excess number of negative ions. A 3rd departure betwixt primary grouping and transition-metallic ions is the ease with which they course stable compounds with neutral molecules, such every bit water or ammonia. Salts of main group metal ions dissolve in water to form aqueous solutions. When we let the water evaporate, we get back the original starting material, NaCl(due south). Salts of the transition-metal ions can display a very different behavior. Chromium(Three) chloride, for example, is a violet compound, which dissolves in liquid ammonia to course a yellowish compound with the formula CrCl3 six NH3 that can be isolated when the ammonia is allowed to evaporate. CrCl3(s) + 6 NH3(l) The Electron Configuration of Transition-Metal Ions The relationship betwixt the electron configurations of transition-metal elements and their ions is complex. Example: Let's consider the chemistry of cobalt which forms complexes that comprise either Co2+ or Co3+ ions. The electron configuration of a neutral cobalt cantlet is written as follows. Co: [Ar] 4south 2 3d 7 The discussion of the relative energies of the diminutive orbitals suggests that the ivs orbital has a lower energy than the 3d orbitals. Thus, we might expect cobalt to lose electrons from the higher energy 3d orbitals, merely this is not what is observed. The Cotwo+ and Co3+ ions have the post-obit electron configurations. Cotwo+: [Ar] iiid 7 Co3+: [Ar] 3d vi In general, electrons are removed from the valence-trounce southward orbitals before they are removed from valence d orbitals when transition metals are ionized. Considering the valence electrons in transition-metallic ions are full-bodied in d orbitals, these ions are often described as having d north configurations. The Co3+ and Feii+ ions, for example, are said to have a d half dozen configuration. Coiii+: [Ar] threed 6 Fe2+: [Ar] 3d 6 Oxidation States of the Transition Metals Most transition metals form more than than one oxidation state. Some oxidation states, however, are more common than others. The most common oxidation states of the offset series of transition metals are given in the tabular array beneath. Efforts to explicate the apparent pattern in this table ultimately fail for a combination of reasons. Some of these oxidation states are common because they are relatively stable. Others describe compounds that are not necessarily stable but which react slowly. Nonetheless others are common only from a celebrated perspective. Mutual Oxidation States of the First Series of Transition Metals Ane point almost the oxidation states of transition metals deserves particular attention: Transition-metallic ions with charges larger than +3 cannot be in aqueous solution. Consider the following reaction in which manganese is oxidized from the +ii to the +vii oxidation state. Mn2+(aq) + 4 H2O(l) When the manganese atom is oxidized, it becomes more electronegative. In the +seven oxidation land, this atom is electronegative enough to react with water to form a covalent oxide, MnO4 -. Information technology is useful to have a way of distinguishing between the accuse on a transition-metal ion and the oxidation land of the transition metal. By convention, symbols such equally Mnii+ refer to ions that carry a +ii charge. Symbols such as Mn(VII) are used to depict compounds in which manganese is in the +7 oxidation country. Mn(Vii) is not the only case of an oxidation country powerful enough to decompose water. Equally soon as Mnii+ is oxidized to Mn(Iv), information technology reacts with water to class MnOii. A similar phenomenon tin can be seen in the chemistry of both vanadium and chromium. Vanadium exists in aqueous solutions as the 5ii+ ion. But in one case it is oxidized to the +four or +five oxidation state, it reacts with water to form the VO2+ or VO2 + ion. The Cr3+ ion tin can be institute in aqueous solution. But once this ion is oxidized to Cr(VI), it reacts with water to form the CrO4 2- and Cr2O7 2- ions.
![]()
HiiO NaCl(s) ![]()
Na+(aq) + Cl-(aq)
![]() CrCliii 6 NH3(s)
CrCliii 6 NH3(s) ![]()
![]()
Sc Ti V Cr Mn Fe Co Ni Cu Zn +1 d 10 +2 d 3 d 5 d half-dozen d seven d eight d ix d 10 +3 d 0 d three d 5 d 6 +4 d 0 d iii +5 d 0 +half dozen d 0 +seven d 0 ![]() MnO4 -(aq) + eight H+(aq) + v e-
MnO4 -(aq) + eight H+(aq) + v e- ![]()
Source: https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch12/trans.php
0 Response to "How Do You Know Charge of Transition Metals"
ارسال یک نظر